Registration Of Drug Product In Malaysia (2025)

Overview
In Malaysia, drug product registration is regulated by the National Pharmaceutical Regulatory Agency (NPRA) under the Ministry of Health Malaysia (MOH). All pharmaceutical products intended for importation, manufacture, distribution, or sale must be registered with the NPRA to ensure their safety, efficacy, and quality.
The registration dossier must follow the ICH CTD/ASEAN Common Technical Dossier (ACTD) format, which includes administrative, quality, non-clinical, and clinical documentation. Only a company that is locally registered with the Drug Control Authority (DCA) and holds a valid Manufacturer’s License or Import License can act as the Marketing Authorization Holder (MAH).
The NPRA conducts administrative screening and technical evaluation which includes:
Product formulation and quality assessment
GMP (Good Manufacturing Practice) verification
Bioequivalence studies for generic medicines
Safety and efficacy review for new chemical entities (NCEs) and biologics
For imported drugs, supporting documents such as the Certificate of Pharmaceutical Product (CPP), GMP certificate, and Letter of Authorization from the product owner are mandatory. All product labels and package inserts must comply with NPRA guidelines and include Bahasa Malaysia and English where required.
Once approved, the product is issued a Registration Number. The validity of the product registration is 5 years, subject to renewal and adherence to post-marketing surveillance requirements such as adverse event reporting, variations, and recalls if needed.
Registration Process
|
|
|
|
Register as Pre-Membership User
The Pre-Membership module allows unregistered users to access online forms. This page is intended for users who are not yet full members and need access to pre-membership forms linked to the QUEST3+ system.
To conduct transactions for product registration, variation, licensing, renewal and other related transactions, you must register your full membership and purchase your digital certificate in the form of USB token. Once you have obtained and installed your digital certificate, you will be able to conduct your transactions in QUEST 3+.
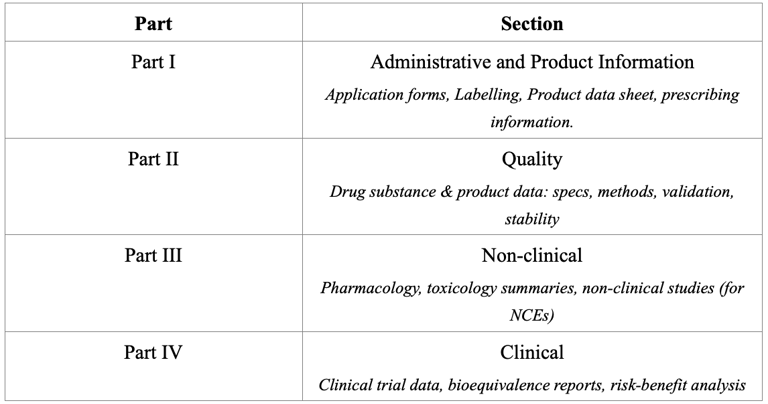
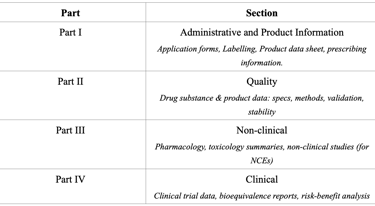
Registration Dossier Structure
Malaysia adopts the ICH CTD/ASEAN Common Technical Dossier (ACTD) format for drug registration. The dossier consists of four parts, each covering a specific set of data.
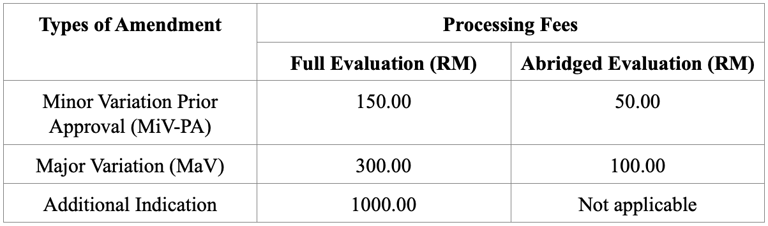
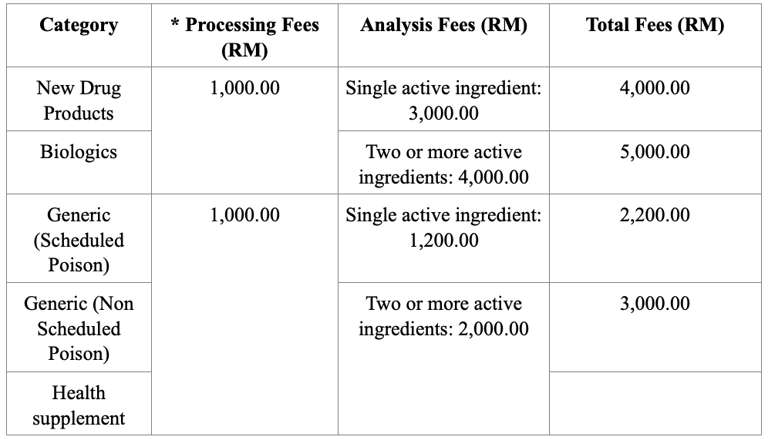
Registration Fees
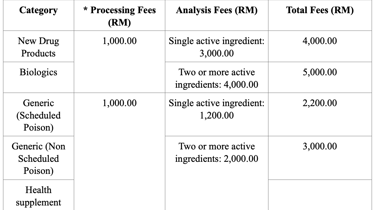
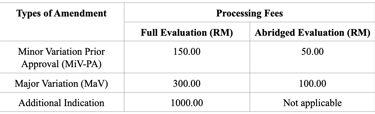
Variation and Additional Indication