Registration Of Drug Product In Singapore (2025)
Overview
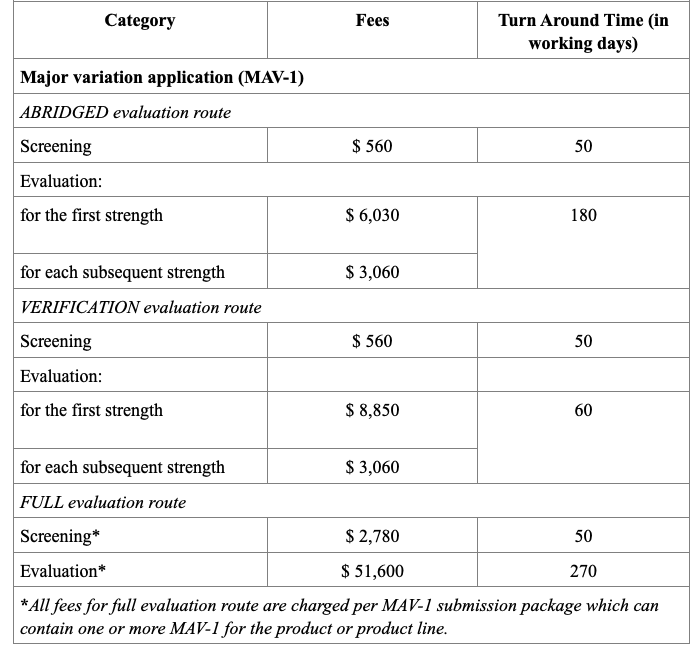
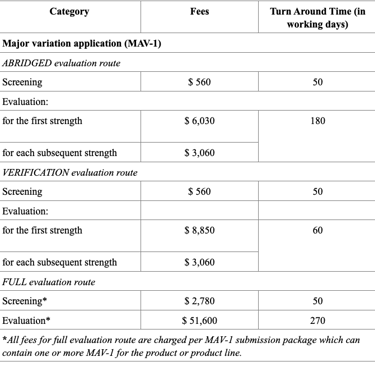
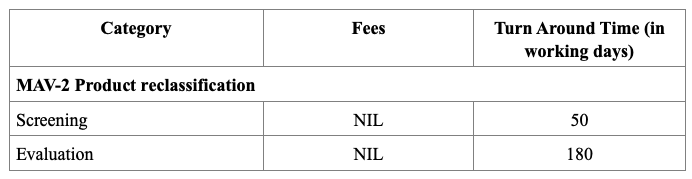
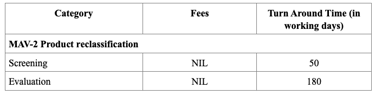
In Singapore, the registration of drug products is regulated by the Health Sciences Authority (HSA), under the Ministry of Health. The process requires submission of a Common Technical Document (CTD)-formatted dossier, comprising Modules 1 to 5 or ACTD format. Module 1 contains Singapore-specific administrative documents such as the application form, GMP certificate, product labels, and Certificates of Pharmaceutical Product (CPP), all of which must be submitted through the HSA’s online portal known as PRISM. Depending on the product’s regulatory status in other countries, applicants may pursue one of three evaluation pathways: Full, Abridged, or Verification. The Full Evaluation route applies to new drug entities, while the Abridged and Verification routes are available for products already approved by HSA-recognized reference agencies (e.g., US FDA, EMA, TGA).
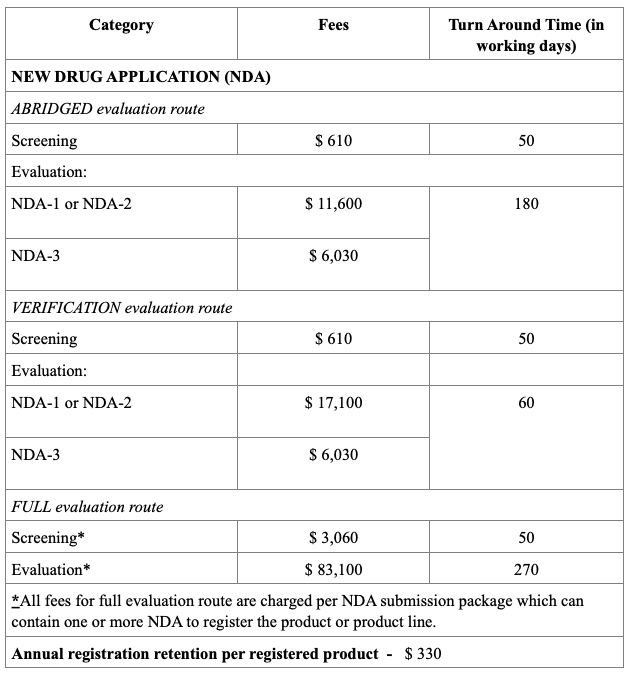
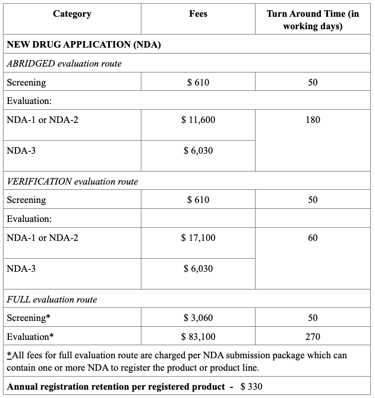
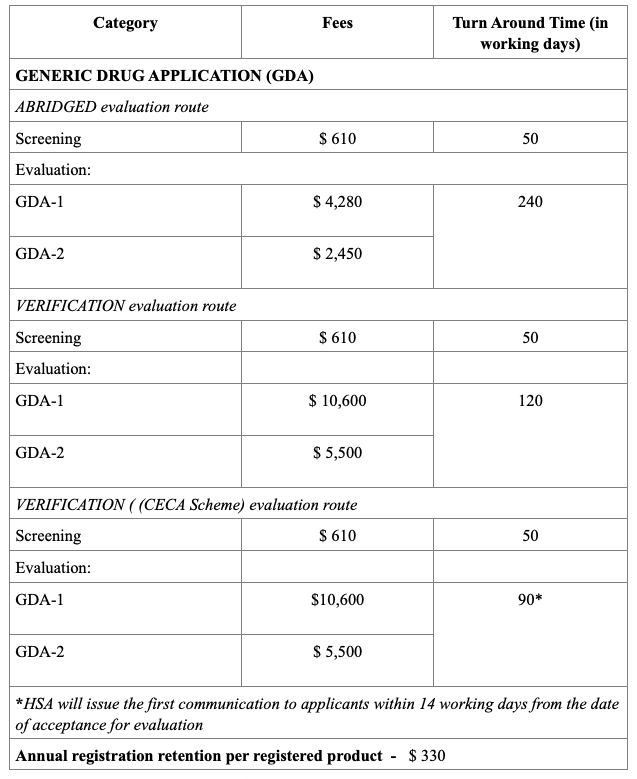
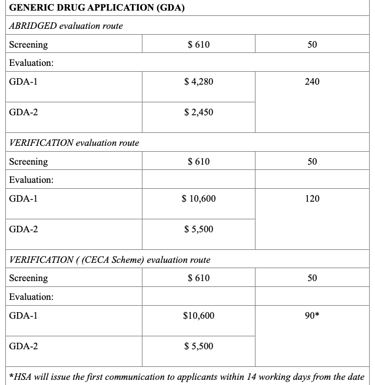
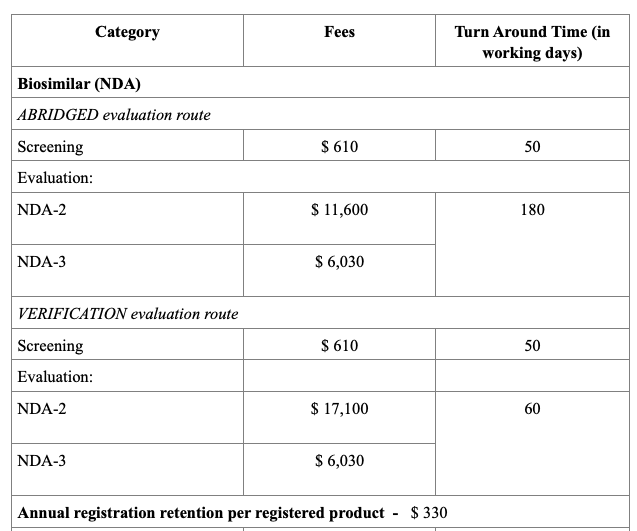
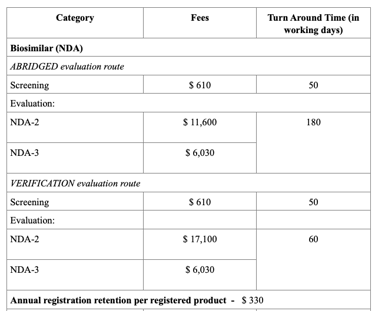
The evaluation process typically includes an administrative screening followed by scientific assessment, which may occur in one or two rounds. Timelines vary from approximately 60 to 270 working days, based on the route chosen. Registration fees also differ, with Full Evaluation generally being the costliest. After approval, any changes to the product must be submitted as variations, and license renewals and safety monitoring are required to maintain market authorization.


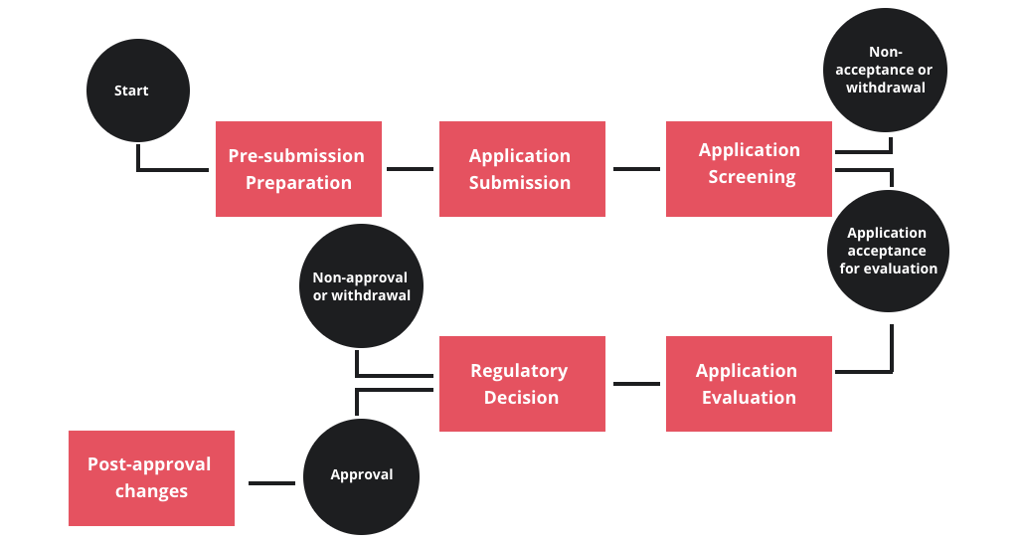
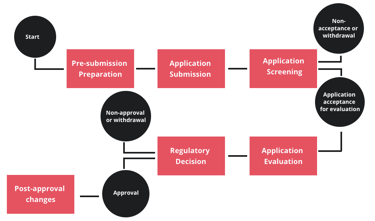
Registration Process
Registration Dossier Structure
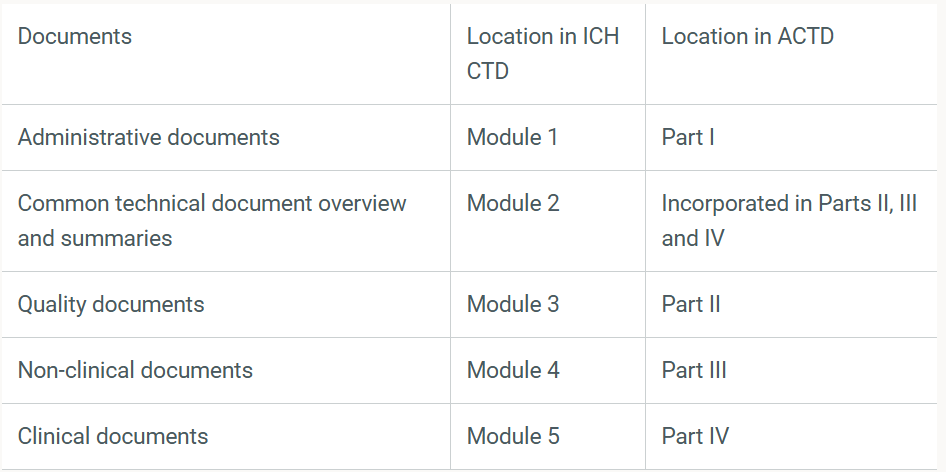
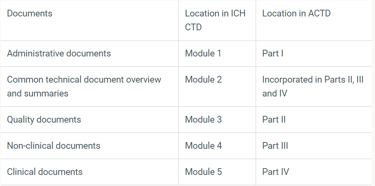
Applicants are required to organise their dossiers following the Common Technical Document (CTD) format as defined by the International Council for Harmonisation (ICH) or the ASEAN CTD format.
Note:
The CTD format cannot be changed once the application is submitted.
Any subsequent variation applications for the product should follow the same format.
Eligibility and Application Process
To register therapeutic products in Singapore, company must be locally incorporated and registered with ACRA.
To submit your application, access the HSA's online platform via PRISM (Pharmaceutical Regulatory Information System). Before starting the submission, ensure you have the following in place:
An active CRIS (Client Registration and Identification Service) company account.
Either a Corppass login or an HSA PIN for secure access.
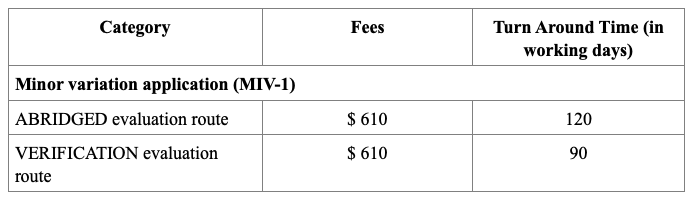
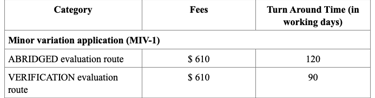
Registration Fees
MIV-2 applications (Notification or Do-and-Tell)
There are no fees for MIV-2 applications.
MIV-2 (Notification) changes can be implemented 40 working days after application submission, if there are no objections from us.
MIV-2 (Do-and-Tell) applications do not require prior approval but must be submitted to us within 6 months of the implementation of changes.
Start
Pre-submission Preparation
Application Submission
Application Screening
Non-acceptance or withdrawal
Application acceptance for evaluation
Application Evaluation
Regulatory Decision
Non-approval or withdrawal
Approval
Post-approval changes