Structured Approach to receive EUGMP
A Step-by-Step Guide to Achieving EU GMP Certification
Achieving EU GMP (Good Manufacturing Practice) certification is a crucial milestone for pharmaceutical manufacturers aiming to access the European market. It validates that your facility consistently meets high-quality standards for manufacturing, control, and documentation — ensuring product safety and regulatory confidence.
While the process may seem complex, a structured approach can make the journey seamless and predictable.
Here’s a step-by-step guide to help companies prepare for and achieve EU GMP certification successfully.
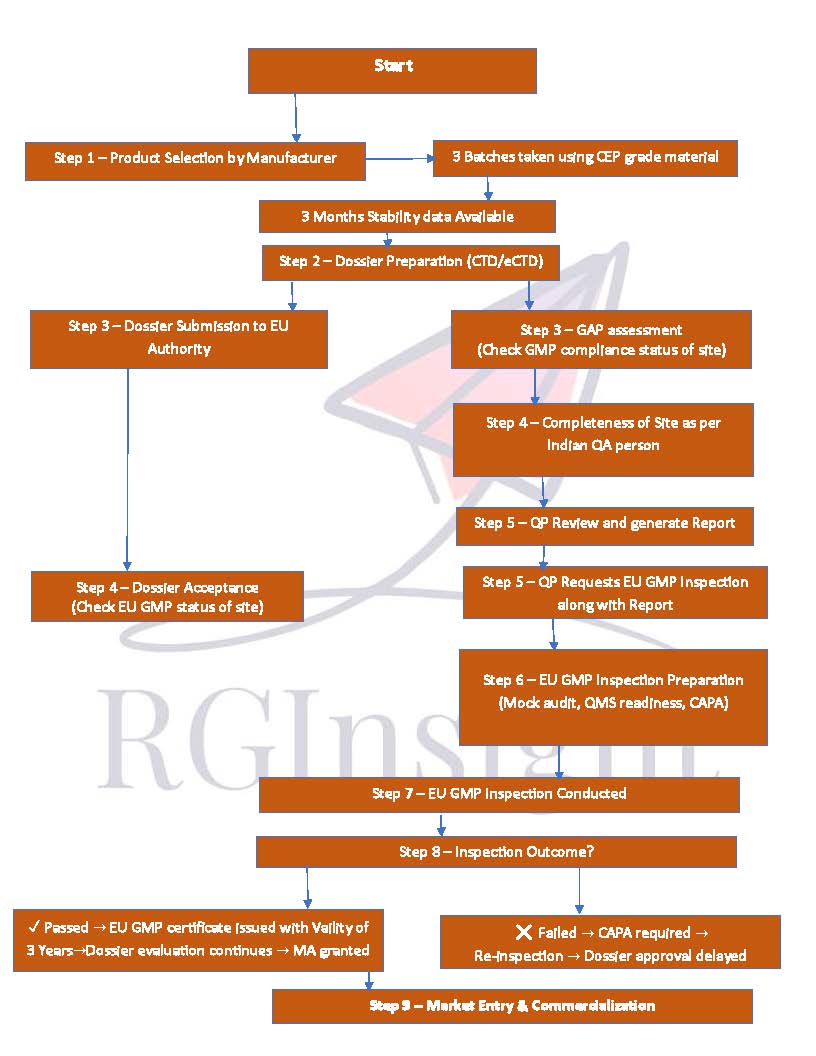
Step 1: GAP Audit – The Foundation
The first step toward EU GMP certification is a GAP Audit.
This involves a detailed evaluation of your facility, systems, and quality processes against EU GMP guidelines.
Key Activities:
Review of the Site Master File (SMF) and supporting documentation
Development of a GAP Audit agenda
On-site audit by experienced EU GMP auditors
Issuance of a GAP Audit Report highlighting non-conformities and improvement areas
After the audit, the manufacturer must prepare a CAPA (Corrective and Preventive Action) plan to address observations. Once the CAPA plan is mutually agreed upon, the project moves to the next stage.
Step 2: CAPA Implementation & Pre-Inspection Review
Following the GAP Audit, the facility must begin implementing the approved CAPA plan to bridge identified gaps.
This stage ensures the site aligns its systems and practices with EU GMP expectations.
Key Activities:
Execution of CAPA actions and documentation updates
Internal verification by RGI’s Indian auditors
Follow-up inspection by EU auditors to confirm compliance readiness
Successful completion of this phase ensures the facility is inspection-ready for the main EU GMP assessment.
Step 3: Dossier Submission & EU GMP Inspection Application
Once the facility is GMP-compliant and ready for inspection, the application process for EU GMP inspection begins.
Key Activities:
Submission of product dossiers to the selected EU authority
Application for EU GMP inspection (which can only be done post-dossier submission)
Coordination to secure a preferred inspection time slot
Submission of PV (Process Validation) batch data and other required information
At this point, the EU authority schedules the inspection, marking a major milestone in the certification process.
Step 4: Main EU GMP Inspection
This is the most critical stage, where EU inspectors assess the facility’s compliance with EU GMP Part I and II guidelines.
Key Activities:
On-site inspection by EU GMP inspectors
Review of production, QC, QA, documentation, and validation systems
Issuance of an Observation Report (if applicable)
Our experts accompany the inspection team to ensure the process runs smoothly and all responses are managed efficiently.
Step 5: CAPA Response & Certification Issuance
If observations are raised during the inspection, the manufacturer must submit a CAPA response addressing each point.
Key Activities:
Draft CAPA prepared by the manufacturer and reviewed by RGI experts
Final submission to the EU authority
Follow-up communication until all corrective actions are accepted
Once the authority is satisfied with the compliance level, the EU GMP Certificate is issued — confirming the facility’s approval to supply products to the EU market.
Step 6: Maintaining EU GMP Compliance
Certification is not the end — it’s the beginning of a continuous journey of quality excellence.
Regular internal audits, documentation updates, and vigilance in regulatory changes are vital to maintaining certification validity.
Final Thoughts
Obtaining EU GMP certification is a significant achievement that strengthens your global credibility, enhances business opportunities, and builds trust among regulatory agencies and partners.


Registration Fees
Fresh Registration
Fee: 5,500,000 VND (~ 220 USD).
Variation Submission (MiV or MaV)
Fee: 1,200,000 VND (~ 48 USD) per variation dossier
5-Year Visa Extension
Fee: 1,500,000 VND (~ 60 USD).
Pre- Submission
Submission
Fee Payment
Post Decision